LTER-Las Madres: basics |
Las Madres lake is a small artificial environment (3.4 ha, average depth: 5.8 m; max depth: 15.5 m; see Table below) which stratifies from March-April until October-November and shows interesting meromictic features. It is a very recent, gravel-pit lake arising from mineral extraction (mostly sand and gravel) carried out in the seventies; alluvial groundwater filled the basin onwards. By 1984 mining has been abandoned. In fact it is a lake complex encompassed by four basins arranged along an E-W axis with groundwater connections. Long-term studies have been carried out in the Eastern basin, which is also the largest one, since 1991. This is located within a sandy pit with steeper slopes at the southern margin. No surficial water inputs occur, other than rainfall; groundwater is the most important water source and lake levels depend upon evaporation and groundwater exchange whereby it can be considered as a seepage lake.
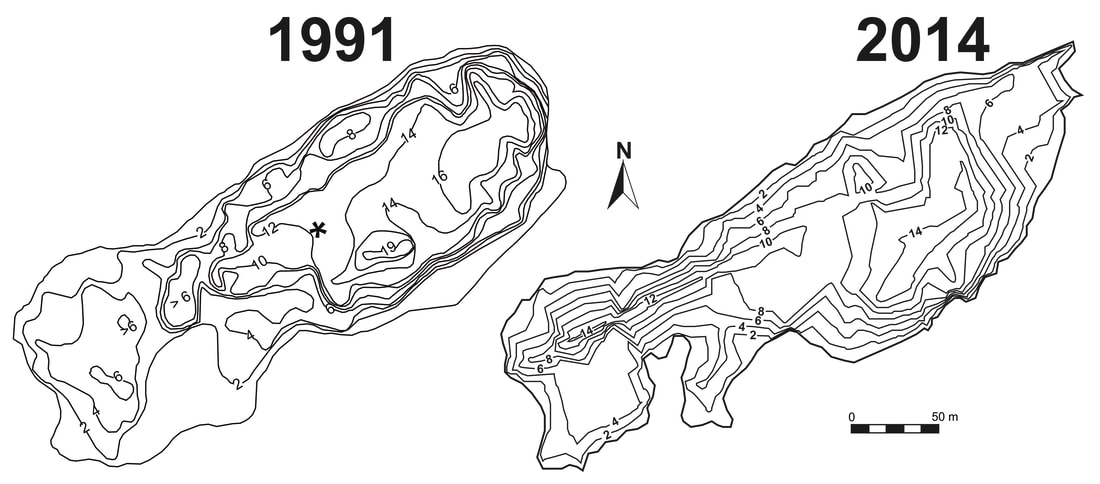
Fig. 1. Bathymetric maps of easternmost basin of Las Madres lake in 1991 and 2014 . Depth contours are shown in meters. For some 20 years, sampling was carried out at the site shown with an asterisk in the 1991 mapl. Later on, it was undertaken within the 14 m isoline on the right of the 2014 picture.
|
Water transparency is highly variable (see Table 1 in "Main features"), fluctuating between 3-5 m during stratification. Both resuspension and an upwelling plume from monimolimnion water can reduce it below 1 m in Nov-Dec some years. The lake is meromictic in most years due to groundwater inputs and nephelometric deep waters with high iron and manganese reduced concentrations that can be oxidized after upwelling, giving an orange colour to surficial lake waters. Deep waters above monimolimnion appear to be greenish due to marl dissolution. Dissolved oxygen is frequently around nil values in bottom waters, whose concentration is impaired by meromictic processes (see below the picture of a core, taken in Sept 2003).
P concentration is low, whereas nitrogen can be relatively high arising from earlier pollution from croplands, which are now discontinued. Following OECD criteria, the lake is oligo-mesotrophic and primary production is limited by phosphorus. Sodium-calcium sulphates prevail in lake waters arising from dissolution of nearby gypsum cliffs. Sulphide stink is sometimes perceived in Summer. Alkalinity uses to be higher than 3.3 meq/L and sulfate transformation by redox processes usually result in high fluctuations. No chemical pollution is observed, except for some hydrocarbon films sometimes coming from an upper lake which is impaired by such pollutants. The nephelometric water layer is also rich in particulate organic matter coming from decaying emergent plants which live in the 2-3 m wide littoral fringe in spring and summer and is encompassed by reed (Phragmites australis), cattail (Typha domingensis) and some scattered Schoenoplectus lacustris stems. In addition to the helophytic fringe, some shallow waters harbour the hydrophyte Najas marina (Gil, 1991). Many insects belonging to Heteropterans, dragonflies and Chironomids can be found in the lake (García-Avilés, 2002a, b; Alvarez-Cobelas, unpublished data) and even freshwater shrimps (Atyaephyra desmaresti) and turtles (Mauremys leprosa) occur in littoral areas. Bacterial plankton shows high richness, as judged by 16S-ARN analyses. Autotrophic picoplankton also occurs, along with Cilitates (Didinium spp. and other species), heterotrophic nanoflagellates (Gymnodinium spp., among others), diatoms (Cyclotella ocellata), dinoflagellates (Peridinium spp.) and green algae (Planctonema and several Chlorococcales; Rojo & Alvarez-Cobelas, 2001). Long-term studies on the phytoplankton community are reported in Benavent-Corai (2015), which provides evidence of climate warming effects on that community and the factors controlling it when decadal cycles can also be detected. Many rotifer species live there, among which Polyarthra spp., Anuraeopsis fisssa, Hexarthra fennica and Filinia hofmanni can be found (Velasco et al., 1996). Also the Cladocerans Daphnia longispina and Ceriodaphnia reticulata and the Copepods Arctodiaptomus salinus and Tropocyclops prasinus prevail in zooplankton (Alvarez-Cobelas et al., 2006b). More information on the lake and its ecological features is reported by Alvarez-Cobelas & Sánchez-Carrillo (2019). |
The early fish community was encompassed by Cyprinus carpio, Gambusia holbrooki, Lepomis gibbosus and Micropterus salmoides (García-Avilés et al., 1999). In 2016, some barbels were recorded in addition to those fish.
Waterfowl does not breed there, but some ducks can be seen very often. There is a flock of some 30 domestic geese living around. Further avian recordings can be found in García-Avilés et al. (1999).
Some references
Alvarez-Cobelas, M. 2006. Groundwater-mediated limnology in Spain. Limnetica 25: 107-122.
Alvarez-Cobelas, M. & S. Sánchez-Carrillo (eds.) 2019. Ecología acuática de Madrid. CSIC. Madrid.
Alvarez, M., P. Riolobos, Y. Himi, S. Sánchez Carrillo, J. García-Avilés & J. Hidalgo. 2000. Estudio físico-químico de los ambientes estancados del Parque Regional del Sureste de la Comunidad de Madrid. Serie Documentos nº 29. Consejería de Medio Ambiente, Comunidad Autónoma de Madrid. Madrid. 65 pp + 1 disquete.
Alvarez-Cobelas, M., A. Baltanás, J.L. Velasco & C. Rojo. 2002. Daily variations in the optical properties of a small lake. Freshwater Biology 47: 450-461.
Alvarez-Cobelas, M., J.L. Velasco, M. Valladolid, A. Baltanás & C. Rojo. 2005. Daily patterns of mixing and nutrient concentrations during early autumn circulation in a small sheltered lake. Freshwater Biology 50: 813-829.
Alvarez-Cobelas, M., C. Rojo, J.L. Velasco & A. Baltanás. 2006a. Factors controlling planktonic size spectral responses to autumnal circulation in a Mediterranean lake. Freshwater Biology 51: 131-143.
Alvarez-Cobelas, M., A. Baltanás, J.L. Velasco & C. Rojo. 2006b. Zooplankton dynamics during autumn circulation in a small, wind-sheltered, Mediterranean lake. Marine and Freshwater Research 57: 441-452.
Benavent-Corai, J. 2015. Efectos ambientales a largo plazo sobre el fitoplancton de la laguna de Las Madres (Madrid). Ph. D. Thesis. Univ. Valencia, Spain.
García-Avilés, J. 2002a. Biodiversidad de los humedales del Parque Regional del Sureste. II. Libélulas. Serie Documentos nº 36. Centro de Investigaciones Ambientales de la Comunidad de Madrid “Fernando González Bernáldez”. Madrid. 60 pp.
García-Avilés, J. 2002b. Biodiversidad de los humedales del Parque Regional del Sureste. III. Heterópteros acuáticos. Serie Documentos nº 37. Centro de Investigaciones Ambientales de la Comunidad de Madrid “Fernando González Bernáldez”. Madrid. 62 pp.
García-Avilés, J., N. Roblas & J. Hidalgo. 1999. Biodiversidad de los humedales del Parque Regional del Sureste. I. Vertebrados acuáticos. Serie Documentos nº 29. Consejería de Medio Ambiente. Comunidad Autónoma de Madrid. Madrid. 65 pp.
Gil, M. 1991. Notas sobre plantas acuáticas madrileñas, I. Anales del Jardín Botánico de Madrid 49: 292-293.
Rojo, C. & M. Álvarez-Cobelas. 2001. Phytoplankton structure and dynamics at daily temporal scale: response to the thermal overturn. Archiv für Hydrobiologie 151: 549-569.
Rojo, C., K.T. Kiss, M. Alvarez-Cobelas & M.A. Rodrigo. 1999. Population dynamics of Cyclotella ocellata (Bacillariophyceae): endogenous and exogenous factors. Archiv für Hydrobiologie 145: 479-495.
Velasco, J.L., M. Alvarez-Cobelas & A. Rubio. 1996. Influencia de la ruptura de la termoclina sobre la comunidad de rotíferos planctónicos de una laguna meromíctica (Las Madres, Madrid). Ecología 10: 523-532.
Waterfowl does not breed there, but some ducks can be seen very often. There is a flock of some 30 domestic geese living around. Further avian recordings can be found in García-Avilés et al. (1999).
Some references
Alvarez-Cobelas, M. 2006. Groundwater-mediated limnology in Spain. Limnetica 25: 107-122.
Alvarez-Cobelas, M. & S. Sánchez-Carrillo (eds.) 2019. Ecología acuática de Madrid. CSIC. Madrid.
Alvarez, M., P. Riolobos, Y. Himi, S. Sánchez Carrillo, J. García-Avilés & J. Hidalgo. 2000. Estudio físico-químico de los ambientes estancados del Parque Regional del Sureste de la Comunidad de Madrid. Serie Documentos nº 29. Consejería de Medio Ambiente, Comunidad Autónoma de Madrid. Madrid. 65 pp + 1 disquete.
Alvarez-Cobelas, M., A. Baltanás, J.L. Velasco & C. Rojo. 2002. Daily variations in the optical properties of a small lake. Freshwater Biology 47: 450-461.
Alvarez-Cobelas, M., J.L. Velasco, M. Valladolid, A. Baltanás & C. Rojo. 2005. Daily patterns of mixing and nutrient concentrations during early autumn circulation in a small sheltered lake. Freshwater Biology 50: 813-829.
Alvarez-Cobelas, M., C. Rojo, J.L. Velasco & A. Baltanás. 2006a. Factors controlling planktonic size spectral responses to autumnal circulation in a Mediterranean lake. Freshwater Biology 51: 131-143.
Alvarez-Cobelas, M., A. Baltanás, J.L. Velasco & C. Rojo. 2006b. Zooplankton dynamics during autumn circulation in a small, wind-sheltered, Mediterranean lake. Marine and Freshwater Research 57: 441-452.
Benavent-Corai, J. 2015. Efectos ambientales a largo plazo sobre el fitoplancton de la laguna de Las Madres (Madrid). Ph. D. Thesis. Univ. Valencia, Spain.
García-Avilés, J. 2002a. Biodiversidad de los humedales del Parque Regional del Sureste. II. Libélulas. Serie Documentos nº 36. Centro de Investigaciones Ambientales de la Comunidad de Madrid “Fernando González Bernáldez”. Madrid. 60 pp.
García-Avilés, J. 2002b. Biodiversidad de los humedales del Parque Regional del Sureste. III. Heterópteros acuáticos. Serie Documentos nº 37. Centro de Investigaciones Ambientales de la Comunidad de Madrid “Fernando González Bernáldez”. Madrid. 62 pp.
García-Avilés, J., N. Roblas & J. Hidalgo. 1999. Biodiversidad de los humedales del Parque Regional del Sureste. I. Vertebrados acuáticos. Serie Documentos nº 29. Consejería de Medio Ambiente. Comunidad Autónoma de Madrid. Madrid. 65 pp.
Gil, M. 1991. Notas sobre plantas acuáticas madrileñas, I. Anales del Jardín Botánico de Madrid 49: 292-293.
Rojo, C. & M. Álvarez-Cobelas. 2001. Phytoplankton structure and dynamics at daily temporal scale: response to the thermal overturn. Archiv für Hydrobiologie 151: 549-569.
Rojo, C., K.T. Kiss, M. Alvarez-Cobelas & M.A. Rodrigo. 1999. Population dynamics of Cyclotella ocellata (Bacillariophyceae): endogenous and exogenous factors. Archiv für Hydrobiologie 145: 479-495.
Velasco, J.L., M. Alvarez-Cobelas & A. Rubio. 1996. Influencia de la ruptura de la termoclina sobre la comunidad de rotíferos planctónicos de una laguna meromíctica (Las Madres, Madrid). Ecología 10: 523-532.
